
The Versafitcup Double Mobility is based on the original Dual Mobility design, with more than 30 years of successful clinical history. In fact, this concept was first proposed by Prof. Bousquet and the Medical School of St. Etienne, France, back in 1976. The introduction of the Highcross (cross-linked polyethylene by Medacta) Double Mobility liner drastically reduces the wear rate, reproducing performances comparable to Ceramic-on-Ceramic and Metal-on-Metal bearing combinations.
.png)
WHY CHOOSE A DOUBLE MOBILITY CUP?
Better than standard fixed liner cups:
Extremely low wear rate thanks to the Dual Mobility and Third Articulation.
Low dislocation rate thanks to increased head diameter.
High Range of Motion (ROM) thanks to increased head/neck ratio.
The question of a potential UHMWPE wear increase due to the double articulation has been raised, and clinical follow up from more than 30 years on similar systems has been reassuring. Additionally the dynamic mechanism of action is becoming better understood.
The Dual Mobility and Third Articulation concepts are responsible for the low wear rate of the Versafitcup Double Mobility:

Head/Liner Articulation (A1)
A1 is always smaller than A2. Under physiological loading conditions, Al is the first to be mobilized up to contact of stem neck with UHMWPE liner rim. During 80% of one gate cycle the only movement is A1.

Liner/Shell Articulation (A2)
A2 starts only when the stem neck enters into contact with the rim of the liner. The rim of the liner is chamfered to articulate with polished neck (Third Articulation concept). The movement A2 happens with activities requiring high Range Of Motion (ROM) such as climbing stairs.
Thanks to this concept, the wear rate of the Versafitcup is lower compared with standard fixed UHMWPE liner coupled with metal head[8].
By using a Ceramic head instead of a Cobalt Chrome head, the wear rate can be significantly reduced[9].
It is important to always couple the Versafitcup Double Mobility with Medacta femoral stems which have a highly polished neck and a short taper design.
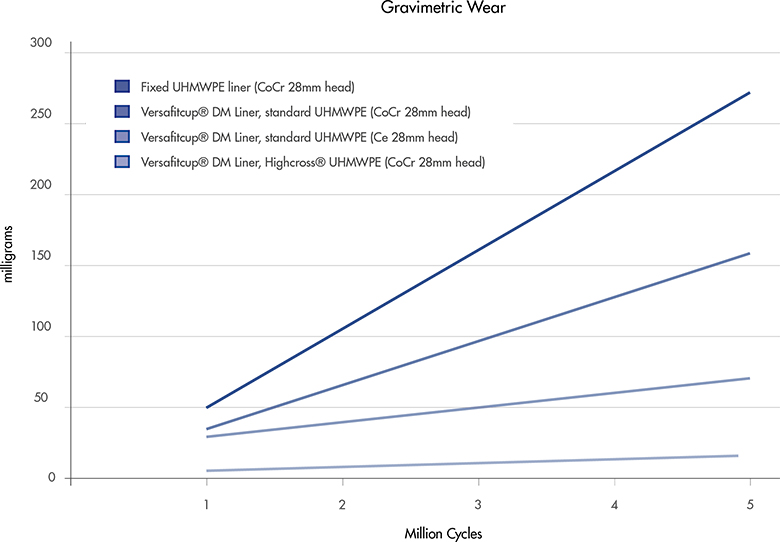
Test[10] on Versafitcup Double Mobility size 64 mm, coupled with standard and Highcross liners, CoCr 28 mm head, 5 Million Cycles with 3400 N according to ISO 14242-1. Other data are an extrapolation from literature[9] and the above mentioned test.
VERSAFITCUP HIGHCROSS DOUBLE MOBILITY LINER
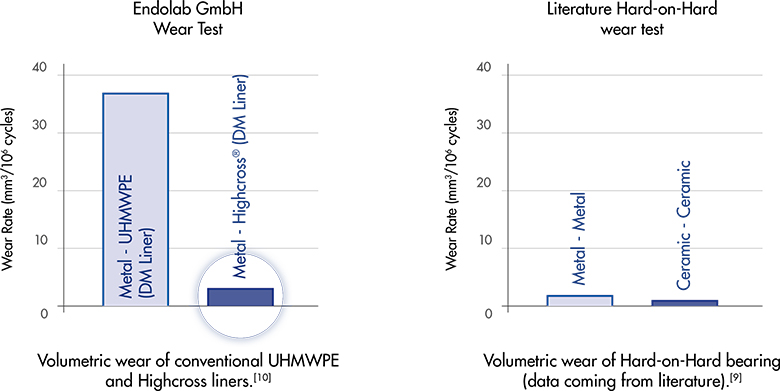
The Versafitcup Double Mobility design provides a low wear rate. The usage of Highcross, cross-linked polyethylene by Medacta, guarantees a further drastic reduction of wear production. In fact, Highcross Double Mobility liners show a 94% reduction in volumetric wear rate versus the same liners fabricated of ETO sterilized, conventional UHMWPE, thanks to the enhanced properties of cross-linked polyethylene [10].
HIGHCROSS by Medacta is characterised by:
gamma irradiation at 100 kGy,
stabilisation temperature of 150°C,
controlled cooling rate in order to optimise the cristallinity of the material,
final sterilisation with ethylene oxide.
Such innovative production characteristics yield a high quality material with enhanced properties.
It has been demonstrated that for Metal-on-Metal bearing, the prosthesis components positioning is very critical and a poor positioning can lead to early failures due to excessive metal debris [12,13].
Also in the case of Ceramic-on-Ceramic bearing, the implants positioning is really critical, in fact a poor positioning can augment the risk of liner fracture or squeaking[14].
The Versafitcup Double Mobility eliminates all these issues, adding additional protection against dislocation, which is the most common cause of Total Hip Arthroplasty revision, according to a recent market survey [15].
When the Versafitcup Double Mobility is implanted, the liner diameter provides the hip joint stability, not the head diameter.

The diameter responsible for hip stability is the Head Diameter (28mm, 32mm, 36mm,…).

The diameter responsible for hip stability is the Liner Diameter (38mm, 40mm, 42mm,…), due to the retentive mechanism for the femoral head.
In the Versafitcup product range, the minimal diameter of the liner is 38mm for the 46mm cup and increases with 2 mm steps up to 56mm for the 64mm cup. (Liners can be coupled with 28 mm head for acetabular shells from sizes 48 mm to 64 mm; 22 mm head coupling available for shell sizes 46 mm and 48 mm).

Thanks to that concept, the clinical results reported in the literature on similar product systems show extremely low dislocation rates as low as 0.1% with over 1000 patients enrolled [4,5,6,7].
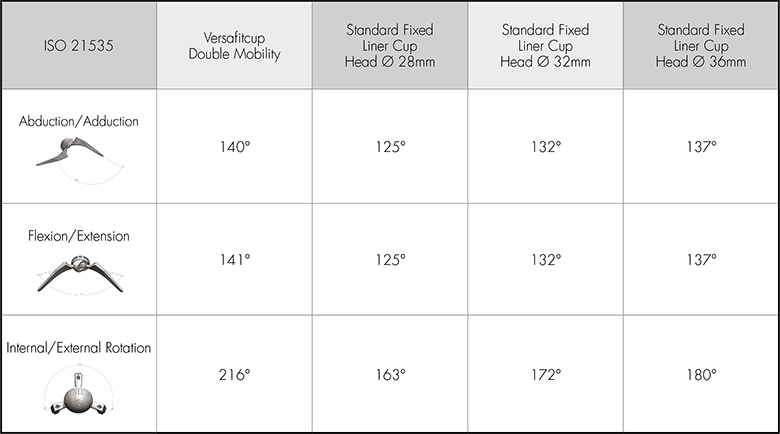
The table compares the ROM of the Versafitcup Double Mobility with a standard fixed liner cup size 64mm with different head sizes (28mm, 32mm, 36mm).
Thanks to the Dual Mobility concept, the Versafitcup has a Head/Neck ratio close to 3.25 for the smallest size increasing to 4.80 for the biggest size; thus providing the high Range of Motion of the Versafitcup Double Mobility.
THE DUAL MOBILITY CONCEPT: 30 YEARS OF EXCELLENT CLINICAL RESULTS
The dual mobility concept was first proposed by Prof. Gilles Bousquet in 1976. The basic idea is to couple two concepts: decrease wear according to the low friction concept of Charnley and achieve an intrinsic stability of the articulation utilizing a femoral head of bigger size, more similar to patient’s anatomy as advocated by McKee-Farrar.
The system conceived by Bousquet is basically composed of a press-fit acetabular shell, realizing two distinct concentric articulations:
The UHMWPE liner represents, according to the different sizes, approximately 5/8 of a sphere and it is invariably designed with a retentive mechanism for the femoral head.
Stress reduction has been observed and documented over the years and at least three publications of Aubriot et al.[1], Farizon et al.[2] and Leclercq et al.[3] have been dealing with this issue. Altogether more than 380 patients have been followed up for a period of more than 10 years. Results were excellent with an implant’s survival curve exceeding 95%.
Even in those cases where a mobilization of the implant led to revision, this was never in association with loss of bone stock suggesting an optimal distribution of stresses.
Several other authors have been addressing the issue of implant stability, early dislocation in relation to a double mobility or bi-articular cup [4, 5, 6, 7].
Early dislocation remains the main complication after total hip replacement surgery, and its origin is most often multifaceted including surgical mistakes, errors in orientation or lateralisation of the implants, length of the limb, muscular insufficiency, lever effect, neurological disturbances, etc. In the above mentioned literature review patients with recidivant dislocation or at high risk of dislocation have been satisfactorily treated with this kind of implant.
In terms of safety, Leclercq et al. [6] reports only 1 dislocation on 1100 implants over a period of more than 10 years, which corresponds to a percentage of 0.1%.
Clinical studies on Versafitcup Double Mobility demonstrated that after a minimum follow-up of 5 years there have been no dislocation, no cup migration, no loosening, no detectable wear and no revision surgery. [16]
In the Versafitcup Double Mobility product range, the minimal diameter of the liner is 38mm for the 46mm cup and increases with 2 mm steps up to 56mm for the 64mm cup. (Liners can be coupled with 28 mm head for acetabular shells from sizes 48 mm to 64 mm; 22 mm head coupling available for shell sizes 46 mm and 48 mm).


The Versafitcup Double Mobility elliptical press-fit cup geometry with equatorial macrostructure and surface effect provides a gradual load transfer avoiding peaks and ensuring excellent primary stability.

The surface treatment consist of:

The equatorial macrostructures shows circular retaining splines which increase the contact between the implant and the bone by 30 to 40%.

An upper edge in the shape of a 5° bill provides an additional cover for the articulating liner.

Solid high nitrogen stainless steel cup, interior mirror polishing, without screw holes. Contains a mechanism for mechanical stability during cup impaction.
The anterior approach, strengthened by several years of clinical experience, is the only technique which follows a path both intermuscular and internervous and therefore reduces considerably the risk of damaging periarticular structures such as muscles, tendons, vessels and nerves.
Thanks to its expertise, unique in the market, Medacta International is the worldwide leader for AMIS (Anterior Minimally Invasive Surgery) with worldwide learning centers to teach the surgical approach, permanent support for surgeons starting with AMIS and continuous development of specific AMIS instrumentation.
With the use of Versafitcup DM you can enter the Medacta International AMIS world.
Discover:


Versafitcup Double Mobility is the valid alternative to Ceramic-on-Ceramic and Metal-on-Metal Large Heads, thanks to its advantages:
The Versafitcup Double Mobility belongs to the Versafitcup system, a complete system of elliptical press-fit cups that shares the same instrumentation.
The system includes the Versafitcup CC Trio, a press-fit cup with lateral screw holes offering the possibility to increase fixation with flat head cancellous bone screws. The Versafitcup CC Trio inner shell has been designed to allow the use of bigger heads to better meet the needs of patients and surgeons and better restore biomechanics.
[1] J.H. Aubriot, P. Lesimple, S. Leclercq, Study on cementless Bousquet type cup in one hundred hybrid total hip replacement (femoral stem cemented Charnley type) Average follow-up 5 years. Acta Orthopaedica Belgica, Vol 59 Suppl.I.-1993, Service de Chirurgie Orthopédique et Traumatologique, CHR Côte de Nacre, Caen, France.
[2] F. Farizon, R. de Lavison et al, Results with a cementless alumina coated cup with a dual mobility, a twelve years follow-up study. Int Orthop. 1998; 22(4) : 219-224.
[3] S. Leclercq, P. Lemaréchal, D. Richter, J. Aubriot, Charnley-Kerboull-Bousquet hybrid THR after 10 years, Charnley 2000 Total Hip Arthroplasty, 3rd International Symposium, Lyon, France.
[4] J. Debeyre, Dislocation of cemented total hip replacement, Revue de Chirurgie Orthopédique, 1975, 61, 39-42.
[5] S. André, P. Feuillhade De Chauvin, F. Tiberi, M. Postel, Dislocation of Charnely type and Charnley modified Kerboull type Total Hip Replacement, Revue de Chirurgie Orthopédique, 1983, 69, 447-453.
[6] S. Leclercq, S. El Blidi, J.H. Aubriot, Treatment of recurrent dislocation of Total Hip Replacement using Bousquet type double mobility cup. Review of 13 cases, Revue de Chirurgie Orthopédique, 81, 389-394, Service de Chirurgie Orthopédique et Traumatologique, CHR Côte de Nacre, Caen, France.
[7] S. Leclercq, P. Lemaréchal, F. Menguy, J. H. Aubriot, Treatment of recurrent dislocation of Total Hip Replacement using Bousquet type double mobility cup, Literature overview. 74ème réunion annuelle de la SOFCOT, Revue de Chirurgie Orthopédique Novembre 1999, Vol 85, Suppl III, Service de Chirurgie Orthopédique et Traumatologique, CHR Côte de Nacre, Caen, France.
[8] Data on file Medacta®.
[9] A.S. Greenwald, J.P. Garino. Alternative bearing surfaces: the good, the bad, and the ugly. J Bone Joint Surg 83-A, Suppl 2 Pt 2: 68-72, 2001.
[10] The UHMWPE raw material, used for both conventional UHMWPE and HighCross™ UHMWPE, is Chirulen 1020. The liners of each were manufactured by turning compression molded UHMWPE bars and were finished products that followed all the manufacturing process flow including final sterilization. The liners tested were 12.9 mm thick, size 64/28. Testing was conducted under a multi-axial hip joint simulation for 5 million cycles using a 28 mm size M CoCrMo ball head and metal actebular shell of size 64 mm. To create a worst case situation, a special attachment collar was utilized to force the liner into contact to simulate impingement with every cycle of the gait. The embedding medium was composed of bovine serum with a 30 g/L protein content and deionized water. It was filtered before doing the test and maintained at 37°C. The serum was replaced every 0.5 million cycles. Three liners of each type were tested. Wear was measured at 0.5, 1, 2, 3, 4 and 5 million cycles. Average volumetric wear rates were 38.3 +/- 2.3 mm3 per 106 cycles for the Versafitcup Double Mobility conventional UHMWPE liners and 2.5 +/- 0.8 mm3 per 106 cycles for the Versafitcup Double Mobility HighCross™ UHMWPE liners. (See plots below) The in vitro hip wear simulator tests have not been shown to quantitatively predict clinical wear performance.
[11] F.Siccardi, I.Quagliana, M.Bernardoni, E.Spadini, M.Zimmermann, Analysis of Versafitcup Double Mobility Wear Rates, Medacta White Paper, January 2010.
[12] R. Trace, Soft tissue reactions to metal-on-metal hip arthroplasty are due mostly to surface wear, Orthopaedics Today Europe 2009; 12:6
[13] D. J. Langton, S. S. Jameson, T. J. Joyce, N. J. Hallab, S. Natu, A. V. F. Nargol, Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement, J Bone Joint Surg [Br] 2010; 92-B:38-46
[14] S. Affatato, F. Traina,C. Mazzega-Fabbro, V.Sergo, M. Viceconti, Is ceramic-on-ceramic squeaking phenomenon reproducible in vitro? A long-term simulator study under severe conditions, J Biomed Mater Res B Appl Biomater. 2009 Oct;91(1):264-71.
[15] K.J. Bozic, The increasing number of THA revisions in the United States: Why is it happening?, Orthopaedics Today Europe 2009; 29:6
[16] P. Laffargue, Dual Mobility, IMUKA 2010, Master class in Hips, February 3rd to 5th, Maastricht (NL)